Introduction: Bleomycin has been an integral part of classical Hodgkin lymphoma (HL) treatment for decades but carries significant toxicity. In particular, acute bleomycin-related lung toxicity occurs in a minority of patients (pts) but has mortality rates as high as 25%. The long-term effects of bleomycin in HL and its impact on lung function are not well known and there is no established guidance for lung screening. The aim of this analysis was to evaluate the impact of bleomycin dose on lung function up to 5 years after treatment in pts recruited to the international, phase 3 RATHL trial.
Methods: RATHL recruited pts with intermediate- or advanced-stage HL aged ≥18 years. Pts received 2 cycles of standard ABVD followed by PET assessment. Pts with complete metabolic response (CMR) received 4 further cycles of chemotherapy, with (ABVD arm; n=469) or without (AVD arm; n=464) bleomycin (12 or 4 doses bleomycin in total, respectively). PET-positive pts switched to BEACOPP-based treatment (total 8-10 doses bleomycin; n=172). Pulmonary function tests (PFTs) were performed at baseline, end of treatment (EOT) and annually for 5 years. Linear regression was used to assess changes in DLCO from baseline and Cox regression used to assess the time to return to within 10% of baseline values, censoring at relapse or death, in all pts, and those with a more than 10% reduction at EOT.
Results: Median age was 33 years (range 18 - 79), 8.2% were aged >60 and 15.1% had a pre-existing respiratory disorder, including asthma in 8.4%. In pts with available data, 18.2% were smokers and 22.2% were ex-smokers with a median of 10 pack-years (IQR: 4 - 20). Baseline DLCO values were low (<75%) in 339 (29.8%) pts and were influenced by disease burden, with lower median DLCO in pts with mediastinal bulk (79%, IQR: 69.0 - 89.4) than in those without (83.1% (73- 94), p=0.006).
Grade ≥3 respiratory adverse events occurred more frequently in the ABVD arm (17 events; 3.6%) than with AVD (7 events; 1.5%), p = 0.041. There was one respiratory death (pulmonary fibrosis) in the ABVD arm.
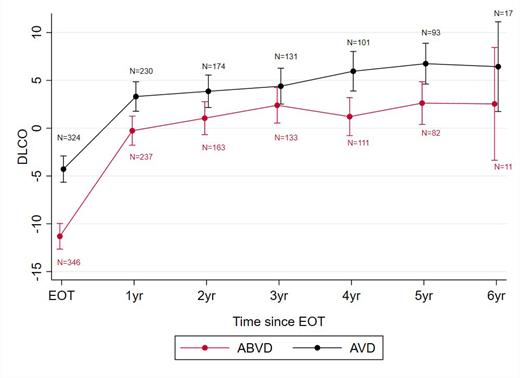
PFT data were available for 779 pts at EOT, 386 pts at 2-year follow up and 196 pts at 5 years; uptake of repeat PFT was unaffected by treatment arm or presence of prior abnormal results. Changes in DLCO adjusted to baseline values for randomised patients are shown in the Figure. Pts randomised to AVD had a significantly smaller reduction in DLCO at EOT compared to those given ABVD or escalated to BEACOPP: +4.1% (95%CI: 1.8 to 6.5, p = 0.001 and +4.1 (0.1 to 8.0) p = 0.043). Other factors associated with a reduction in DLCO were age (-1.6% (-2.4 to -0.8) for a 10-year increase, p<0.001), female sex (-2.7% (-5.0 to -0.44), p=0.019) and baseline pulmonary disease (-4.9 (-8.4 to -1.4), p = 0.006). There was no evidence of an association with abnormal baseline DLCO values. DLCO had reduced to <90% of baseline values for 247 pts (59.8%) in the ABVD arm compared with 167 pts (40.6%; p<0.001) with AVD, and 63 (53.1%) with BEACOPP.
At 2 years after EOT, DLCO had recovered to within 90% of baseline for 69.6% (95% CI: 64.9 - 74.1) of pts in the ABVD arm, 81.4% (77.4 - 85.2) with AVD and 68.5% (59.8 - 76.9) with BEACOPP. DLCO values remained <90% of baseline for 136 pts during follow-up with no reported recovery, with a median PFT follow-up of 18.3 months (IQR: 6.4 - 42.7) after registration. 58 pts had persistent reductions in DLCO at ≥2-year follow up (35 ABVD arm, 10 AVD; 13 BEACOPP arm). Factors associated with a slower recovery of DLCO on univariable analysis were treatment on the ABVD arm (HR 0.71, 95% CI: 0.57 - 0.90; p=0.004 compared with AVD), history of pulmonary disease (HR 0.66 (0.46 - 0.95; p=0.023) and female sex (HR 1.25, 1.01 - 1.54; p=0.041).
DLCO values were consistently higher in the AVD arm than with ABVD throughout follow-up with a mean difference of 3.2% (95% CI: 0.3 to 6.1; p=0.029) at 2 years and 5.0% (1.20 to 8.8; p=0.010) at 5 years, after adjustment for baseline DLCO values (see Figure). Findings were consistent after excluding the minority of pts that received radiotherapy.
Conclusions: Baseline RFTs are influenced by disease burden and do not predict subsequent bleomycin-related changes in lung function. Whilst individual grade ≥3 events were uncommon, there was a population-wide reduction in diffusion capacity that was only partially reversible at 5 years, findings that might have clinical consequences in later years for pts cured of HL. These data strongly support efforts to minimise bleomycin use in the treatment of HL.
Disclosures
Phillips:Takeda: Honoraria, Research Funding; Gilead: Honoraria. Kirkwood:Kite-Gilead: Honoraria. Trotman:Cellectar: Research Funding; BMS: Research Funding; Janssen: Research Funding; Roche: Research Funding; BeiGene: Research Funding. Molin:MSD: Honoraria; BMS: Honoraria; Takeda: Honoraria; Roche: Honoraria. Luminari:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Regeneron Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Radford:ADC Therapeutics, Takeda, Sobi: Consultancy; GlaxoSmithKline: Current equity holder in publicly-traded company; AstraZeneca: Divested equity in a private or publicly-traded company in the past 24 months; Takeda: Research Funding; ADC Therapeutics, Takeda, Sobi: Honoraria; ADC Therapeutics, Takeda: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal